10-Minute Museum: Climate Crisis Mathematics
This is a travelling museum you can visit in only 10 minutes. You learn how mathematical models help us understand global warming and predict changes in the climate.
Ocean Acidification
CO2 is a chemical that can exist as a gas in the atmosphere but also dissolves into water (as in sparkling water), into rocks, or inside living beings. Oceans store about ⅓ of the CO2 on the planet. This is due to a chemical process known as buffering.
Increases in CO2 emissions lead to more CO2 in the oceans. There it reacts with the seawater creating carbonic acid (H2CO3). This raises the acidity of the oceans, affecting sea life. For instance, coral reefs and the shells of marine animals get damaged by it.
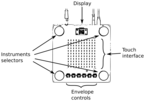
In a display of the 10MM, some sea shells are shown. They have been artificially dissolved in vinegar to illustrate the effect.